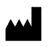| Symbol |
Reference Number of Symbol |
Title of Symbol |
Description of Symbol |
 |
5.1.1 |
Manufacturer |
Indicates the medical device manufacturer |
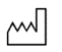 |
5.1.3 |
Date of manufacture |
Indicates the date when the medical device was manufactured. |
 |
5.1.11 |
Country of manufacture |
To identify the country of manufacture of products |
 |
5.1.4 |
Use-by date |
Indicates the date after which the medical device is not to be used. |
 |
5.1.5 |
Batch Code |
Indicates the manufacturer’s batch code so that the batch or lot can be identified. |
 |
5.1.6 |
Catalogue Number |
Indicates the manufacturer’s catalogue number so that the medical device can be identified. |
 |
5.1.7 |
Serial number |
Indicates the manufacturer’s serial number so that a specific medical device can be identified. |
 |
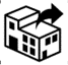
5.1.8 |
Importer |
Indicates the entity importing the medical device into the locale. |
 |
5.1.9 |
Distributor |
Indicates the entity distributing the medical device into the locale. |
 |
5.2.1 |
Sterile |
Indicates a medical device that has been subjected to a sterilization process. |
 |
5.2.3 |
Sterilized using ethylene oxide |
Indicates a medical device that has been sterilized using ethylene oxide. |
 |
5.2.4 |
Sterilized using irradiation |
Indicates a medical device that has been sterilized using irradiation. |
 |
5.2.6 |
Do not resterilize |
Indicates a medical device that is not to be resterilized. |
 |
5.2.7 |
Non-sterile |
Indicates a medical device that has not been subjected to a sterilization process |
 |
5.2.8 |
Do not use if package is damaged and consult instructions for use |
Indicates that a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information. |
 |
5.2.11 |
Single sterile barrier system |
Indicates a single sterile barrier system. |
 |
5.2.12 |
Double sterile barrier system |
Indicates two sterile barrier systems. |
 |
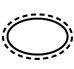
5.2.13 |
Single sterile barrier system with protective packaging inside |
Indicates a single sterile barrier system with protective packaging inside. |
 |
5.2.14 |
Single sterile barrier system with protective packaging outside |
Indicates a single sterile barrier system with protective packaging outside. |
 |
5.3.1 |
Fragile, handle with care |
Indicates a medical device that can be broken or damaged if not handled carefully. |
 |
5.3.2 |
Keep away from sunlight |
Indicates a medical device that needs protection from light sources. |
 |
5.3.4 |
Keep dry |
Indicates a medical device that needs to be protected from moisture. |
 |
5.3.7 |
Temperature limit |
Indicates the temperature limits to which the medical device can be safely exposed. |
 |
5.4.2 |
Do not re-use |
Indicates a medical device that is intended for one single use only. |
 |
5.4.3 |
Consult instructions for use or consult electronic instructions for use |
Indicates need for the user to consult the instructions for use. |
 |
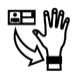
5.4.4 |
Caution |
Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences. |
 |
5.7.2 |
Indicates the name of the patient |
When used, the symbol shall appear adjacent to the patient name or next to a space provided to record it. |
 |
5.7.3 |
Patient identification |
Indicates the identification data of the patient. |
 |
5.7.4 |
Patient information website |
Indicates a website where a patient can obtain additional information on the medical product. |
 |
5.7.5 |
Health care centre or doctor |
Indicates the address of the health care centre or doctor where medical information about the patient may be found. |
 |
5.7.6 |
Date |
Indicates the date that information was entered or a medical procedure took place |
 |
5.7.7 |
Medical device |
Indicates the item is a medical device. |
 |
5.7.10 |
Unique device identifier |
Indicates a carrier that contains unique device identifier information. |
 |
ASTM F2503 |
MR Conditional |
Medical device that has been demonstrated to pose no known hazards in a specified MR environment with specified conditions of use. |
 |
21 CFR 801.109 (b) (1) |
Rx Only |
Federal Law Restricts this Device to Sale by or on the order of a Physician |
 |
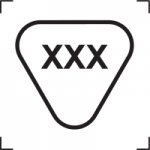
ISO 15223 5.4.9 |
Contains biological material of animal origin |
Indicates a medical device that contains biological tissue, cells, or their derivatives, of animal origin |
 |
ISO 7000/IEC 60417 2725 |
Contains or presence of |
To indicate that the equipment contains the identified product or substance |
 |
ASTM F2503 |
MR Safe |
An item that poses no known hazards resulting from exposure to any MR environment. |